Propane C3H8 Lewis Structure - Propane Threecarbon Alkane Molecular Formula C3h8 Stock ... - And so if we use a #34*l# volume of #dioxygen gas# we will be requiring #(34*l)/5=6.8*l# with respect to #propane gas.#
Propane C3H8 Lewis Structure - Propane Threecarbon Alkane Molecular Formula C3h8 Stock ... - And so if we use a #34*l# volume of #dioxygen gas# we will be requiring #(34*l)/5=6.8*l# with respect to #propane gas.#. 'there are several different strategies to constructing lewis structures. It is a gas at standard temperature and pressure, but compressible to a transportable liquid. Step method to draw lewis structure of propane. Drawing lewis structures can be a straightforward process if the proper steps are followed. Laboratory chemical safety summary (lcss) datasheet.
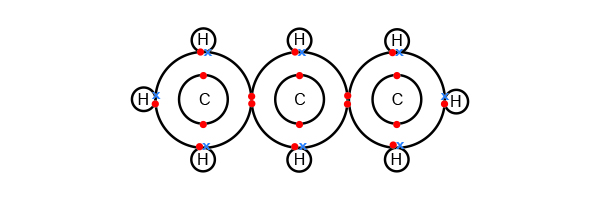
Lewis structure when commonly sold as fuel it is also known as liquified petroleum gas (lpg or lp gas) and is a mixture of propane with smaller amounts of propylene, butane and butylene, plus ethanethiol as an odorant to allow the normally odorless propane to be smelled. The center carbon bonds with itself on each side and two hydrogens. C3h8 contains three carbon atoms, and eight hydrogen atoms. Note that hydrogen only needs two valence electrons to have a full outer shell. What is the lewis structure for c3h8?

Drawing the lewis structure for c3h8 (propane).
And so if we use a #34*l# volume of #dioxygen gas# we will be requiring #(34*l)/5=6.8*l# with respect to #propane gas.# Drawing lewis structures can be a straightforward process if the proper steps are followed. Make a model of propane (c3h8) then draw the structural formula. Before we draw the electron dot diagram, we must first count the number of valence electrons in a molecule of propane. Rate constants can not be estimated for this structure! Is obtained by mixing the hydrocarbon fractions after fractionation of broad fraction of light hydrocarbons. It is a gas at standard temperature and pressure, but compressible to a transportable liquid. Propane pyrolysis plays an important industrial role, mainly in the synthesis of propene, which is used, for example, for producing polypropylene. What is the lewis structure for c3h8? It is a gas at standard temperature and pressure, but compressible to a transportable liquid. What is the name of this structure ch3ch2ch3? The other two carbons bond with 3 hydrogen each. The center carbon bonds with itself on each side and two hydrogens.
It is a gas at standard temperature and pressure, but compressible to a transportable liquid. In the lewis structure for c 3 h 8 there are a total of 20 valence electrons. We besin with so, often molecular formulas will be written in a more clear way like this. Drawing lewis structures can be a straightforward process if the proper steps are followed. After determining how many valence electrons there are in c3h8, place them around the central atom to complete the octets.
In the presence of excess oxygen, propane burns to form water and carbondioxide.
If you are using mobile phone, you could also use menu drawer from browser. 'there are several different strategies to constructing lewis structures. It is a gas at standard temperature and pressure, but compressible to a transportable liquid. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. So we've used all 20 valence electrons for the c3h8 lewis structure. There a total of 20 valence electrons in the lewis structure for c3h8. C3h8 contains three carbon atoms, and eight hydrogen atoms. What are the isomers for c3h8? A video explanation of how to draw the lewis dot structure for propane, along with information about the compound including. The center carbon bonds with itself on each side and two hydrogens. The molecule propane, also known as c3h8, is a linear molecule. Now at constant temperature and pressure, the which we assume, volume is proportional to the number of moles. Propane undergoes combustion reactions in a similar fashion to other alkanes.
In the lewis structure for c 3 h 8 there are a total of 20 valence electrons. Propane (c3h8) is present in nature in petroleum gas and as a minor component in natural gas, the same as ethane but typically in lower proportions. C3h8 lewis structure there is a single bond between each atom. Download image more @ www.chegg.com. 'there are several different strategies to constructing lewis structures.

Laboratory chemical safety summary (lcss) datasheet.
(a) write the lewis structure for propane. 'there are several different strategies to constructing lewis structures. Note that hydrogen only needs two valence electrons to have a full outer shell. This is the c3h8 lewis structure: The center carbon bonds with itself on each side and two hydrogens. Draw lewis structure chcl show unshared. We besin with so, often molecular formulas will be written in a more clear way like this. Drawing the lewis structure for c3h8 (propane). There a total of 20 valence electrons in the lewis structure for c3h8. Before we draw the electron dot diagram, we must first count the number of valence electrons in a molecule of propane. Get the best of about education in your inbox. The structure on the right is the lewis electron structure, or lewis structure, for h 2o. Now at constant temperature and pressure, the which we assume, volume is proportional to the number of moles.
Propane undergoes combustion reactions in a similar fashion to other alkanes c3h8 lewis structure. Propane pyrolysis plays an important industrial role, mainly in the synthesis of propene, which is used, for example, for producing polypropylene.